Categories
New Blog
What Factors Affect The Capacity of A Battery?
October 15 , 2025
What Factors Affect The Capacity of A Battery?
Introduction
Capacity inconsistency among lithium battery cells is one of the key factors leading to pack performance degradation and potential safety risks. These differences arise from multiple sources throughout the battery’s lifecycle — including manufacturing processes, material properties, design parameters, usage conditions, and environmental influences.
This article provides a systematic analysis of the main causes of lithium battery cell capacity variation and offers insights to help improve consistency and reliability in cell production and battery pack integration.
I. Manufacturing Process Factors
(1) Coating and Calendering Deviations
1. Uneven coating thickness
Differences in the coating thickness or density of cathode and anode active materials directly affect the effective reaction area and the amount of lithium-ion intercalation, leading to variations in single-cell capacity. During the coating process, due to the influence of equipment precision and slurry fluidity, the coating thickness may become inconsistent.
For example, in regions where the coating is too thick, the path for lithium-ion intercalation and deintercalation becomes longer, the reaction rate decreases, and thus the cell capacity is affected. Conversely, in regions where the coating is too thin, the effective reaction area of the electrode decreases, also leading to a reduction in capacity.
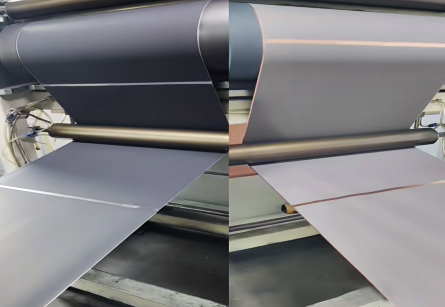
2. Calendering density fluctuations
Excessive compaction may damage the electrode material structure (such as graphite layer breakage), reducing lithium-ion diffusion efficiency; insufficient compaction decreases the amount of active material per unit volume.
During calendering, fluctuations in compaction density affect the electrode porosity and internal resistance. When the compaction density is too high, the porosity of the electrode material decreases, lithium-ion diffusion channels are blocked, and the capacity decreases; when the compaction density is too low, the active material content per unit volume decreases, and the cell capacity is also affected.
(2) Electrolyte Filling and Sealing Defects
1. Differences in electrolyte injection volume
Insufficient electrolyte injection leads to incomplete contact between the electrode sheets and the electrolyte, affecting ion transport rate and causing capacity degradation.
The electrolyte is the medium for lithium-ion transport, and its injection volume directly affects cell performance. If the amount of electrolyte injected is insufficient, the contact area between the electrode sheets and the electrolyte decreases, ion conduction slows down, and the capacity gradually decays.
2. Poor sealing performance
The intrusion of moisture or impurities consumes lithium salts in the electrolyte, destroys the stability of the electrode interface, and aggravates capacity inconsistency.
During sealing, if the tightness is insufficient, external moisture and impurities may enter the cell and react with the lithium salt in the electrolyte, consuming lithium salt and reducing the electrolyte’s performance. Meanwhile, moisture and impurities also damage the stability of the electrode interface, affecting lithium-ion intercalation and deintercalation, thereby worsening capacity inconsistency among cells.
(3) Insufficient Precision in Cell Grading and Sorting
If the cell grading process does not strictly select cells with similar parameters such as capacity and internal resistance, the initial performance of individual cells within a battery pack will be dispersed.
Grading is an important step in the cell production process. Through charge and discharge testing, it screens and matches cells with similar capacity and internal resistance. If the grading precision is insufficient and cells with large differences in these parameters are grouped together, the resulting pack will have inconsistent initial performance, which may cause overcharge or overdischarge issues during use, affecting both the performance and service life of the battery pack.
Our Battery Capacity Grading Machine features high-precision charge/discharge control and automatic sorting, ensuring consistent capacity and resistance matching for superior pack performance.
II. Material and Design Factors
(1) Differences in Electrode Material Performance
1. Cathode materials
For example, in ternary materials (NCM), fluctuations in the nickel, cobalt, and manganese ratios, or crystal structure differences in lithium iron phosphate (LFP), affect the lithium-ion deintercalation capability.
Cathode materials are one of the key factors determining cell capacity. Their performance differences directly affect cell capacity. In ternary materials, different nickel–cobalt–manganese ratios change the crystal structure and electrochemical properties, thus influencing lithium-ion deintercalation. The crystal structure variation of lithium iron phosphate also leads to different electrochemical properties, which in turn affect the cell capacity.
2. Anode materials
Uneven mixing ratios of graphite and silicon-based materials or differences in the expansion coefficient of silicon particles cause different capacity decay rates during cycling.
During charge and discharge, anode materials expand and contract. Their performance differences affect cycle life and capacity consistency. For instance, uneven mixing of graphite and silicon-based materials results in uneven expansion and contraction, which affects cycling performance. Differences in the expansion coefficient of silicon particles cause varying degrees of structural damage to the anode material during cycling, leading to different capacity decay rates.
(2) Material Matching and Formulation Issues
1. Poor compatibility between electrolyte and electrode interface
For example, PC solvents may cause graphite layer exfoliation, or an improper ratio of binder/conductive agent reduces the utilization of active materials.
The compatibility between the electrolyte and electrode interface is a key factor affecting cell performance. Poor compatibility can damage the electrode structure and hinder lithium-ion intercalation and deintercalation, reducing active material utilization. For instance, PC solvent has a high dielectric constant and low viscosity, but it can cause graphite layer exfoliation, harming anode performance. An improper ratio of binder and conductive agent also lowers active material utilization and capacity.
2. Insufficient structural design margin
If adequate capacity redundancy is not reserved in design, low-capacity cells will fail first when manufacturing errors accumulate during cycling.
During cell design, it is necessary to consider manufacturing tolerances and performance degradation, reserving sufficient capacity redundancy. Without enough design margin, low-capacity cells can easily reach overcharge or overdischarge limits first during cycling, resulting in failure and affecting the pack’s overall performance.
III. Usage and Aging Factors
(1) Differences in Cycle Degradation Mechanisms
1. Cathode dissolution and collapse
Long-term charging and discharging cause transition metal ion dissolution, which blocks lithium-ion channels and leads to irreversible capacity loss.
During cycling, structural changes occur in cathode materials, and transition metal ions may dissolve into the electrolyte. These ions can deposit on the anode surface, blocking lithium-ion channels and reducing intercalation/deintercalation efficiency, resulting in irreversible capacity decline.
2. Thickening of the anode SEI film
Repeated cycling causes the solid electrolyte interphase (SEI) film on the anode surface to continuously grow, consuming active lithium and increasing internal resistance, thus reducing effective capacity.
The SEI film protects the anode from further electrolyte reduction, but as cycling continues, it thickens, consuming active lithium and increasing resistance, thereby lowering the effective capacity of the cell.
(2) Lithium Plating and Interface Side Reactions
1. Lithium plating
Low-temperature charging, overcharging, or high-current charging induces lithium deposition on the anode surface, consuming active lithium and accelerating capacity decay.
Under low-temperature, overcharged, or high-current conditions, the intercalation rate of lithium ions on the anode surface may exceed their diffusion rate, causing metallic lithium to deposit—known as lithium plating. This phenomenon consumes active lithium, increases internal resistance, and reduces charge/discharge efficiency.
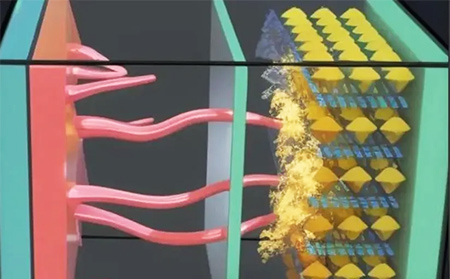
2. Blockage of separator pores by electrolyte decomposition products
Electrolyte decomposition products (e.g., LiF) clog separator pores, impeding ion transport. During cycling, electrolyte decomposition produces byproducts that may block separator pores, hindering ion transport and reducing cell performance.
(3) Effects of Usage History
Overdischarge, high-temperature storage, and other improper operations accelerate cell aging. When new and old cells are mixed, capacity differences become significantly enlarged.
The usage history of cells greatly affects performance and capacity. Improper operations such as overdischarge or long-term high-temperature storage accelerate aging and capacity degradation. Mixing new and aged cells causes large performance differences, increasing capacity variation and the likelihood of overcharge or overdischarge, thereby reducing pack performance and lifespan.
IV. Environmental and External Factors
(1) Uneven Temperature Distribution
Cells located at the edges and center of a pack have different heat dissipation conditions. In high-temperature areas, cell aging accelerates (e.g., electrolyte decomposition rate increases), and capacity decays faster.
Temperature is a critical factor affecting cell performance. In a battery pack, uneven temperature distribution due to varying cooling conditions causes faster degradation in high-temperature regions.
(2) Self-discharge Rate Differences
Cells with different self-discharge rates (e.g., those with higher impurity content) exhibit diverging states of charge (SOC) after long storage, resulting in capacity differences during charging and discharging.
Self-discharge is the spontaneous loss of charge during storage. Differences in self-discharge rates cause SOC divergence, which leads to distinct capacity behavior during use.
(3) Thermal Management Failure
When cooling design is insufficient, local overheating raises internal resistance, reduces charge/discharge efficiency, and lowers capacity utilization.
Thermal management ensures that the pack operates within a suitable temperature range. Inadequate heat dissipation causes local overheating, higher resistance, lower efficiency, and reduced capacity utilization.
V. Conclusion
Cell capacity differences are the result of the combined effects of manufacturing process fluctuations (coating/injection accuracy), intrinsic material properties (electrode active material performance), usage and aging mechanisms (cycle degradation paths), and environmental imbalance (temperature/self-discharge).
To improve the consistency of cell capacity, the following approaches can be taken:
1. Improve manufacturing consistency:
Use high-precision electrode coating machine and automated sorting processes to improve coating and electrolyte injection accuracy. Strictly match cells with similar capacity and internal resistance parameters.
2. Optimize materials and design:
Develop solid-state electrolytes to suppress side reactions, improve electrolyte–electrode compatibility, and enhance electrode material performance.
3. Enhance battery management systems (BMS):
Apply active balancing technology to compensate for capacity differences, monitor the status of each cell in real time, and adjust charge/discharge strategies promptly to ensure pack performance and safety.
In conclusion, in-depth research on the causes of capacity differences in lithium battery cells and the implementation of effective improvement measures are of great significance for enhancing the performance and safety of battery packs.